How are biosimilars developed?
Biosimilar development requires highly trained scientists
Fresenius Kabi has a long-standing heritage in high-quality pharmaceutical development. We are equipped with the necessary global expertise in manufacturing and biotechnology with the goal of achieving high similarity to the reference biologic treatment. Driven by excellence in product quality, Fresenius Kabi biosimilars are manufactured in Europe in compliance with the statutory GMP guidelines.
Biosimilar development requires a deep understanding of the reference product.1 This requires analysis and characterization of the reference product to identify its key characteristics.2
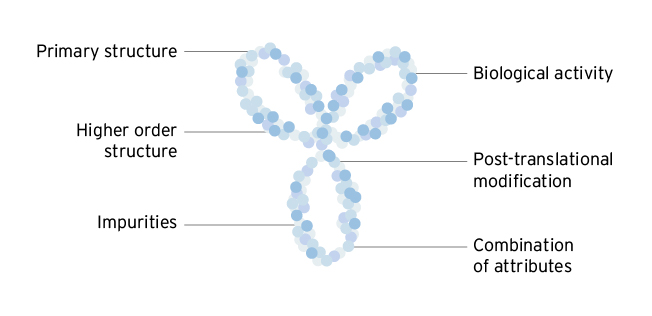
While the primary amino acid sequence of a biosimilar is precisely bioengineered, other features of biologic protein structure such as three-dimensional folding, glycosylation, charge and impurity presence are variable according to the manufacturing process.3 These features of a biologic can affect both the antigen binding and immunogenicity of a drug, and thus can affect drug efficacy and safety.3 Around 40 different analytical methods are used to assess approximately 100 different drug characteristics for biosimilarity between the biosimilar candidate and the reference product.3 These analytical studies are key to the approval process for biosimilars.3
Biosimilars match the reference product in all relevant structural characteristics4
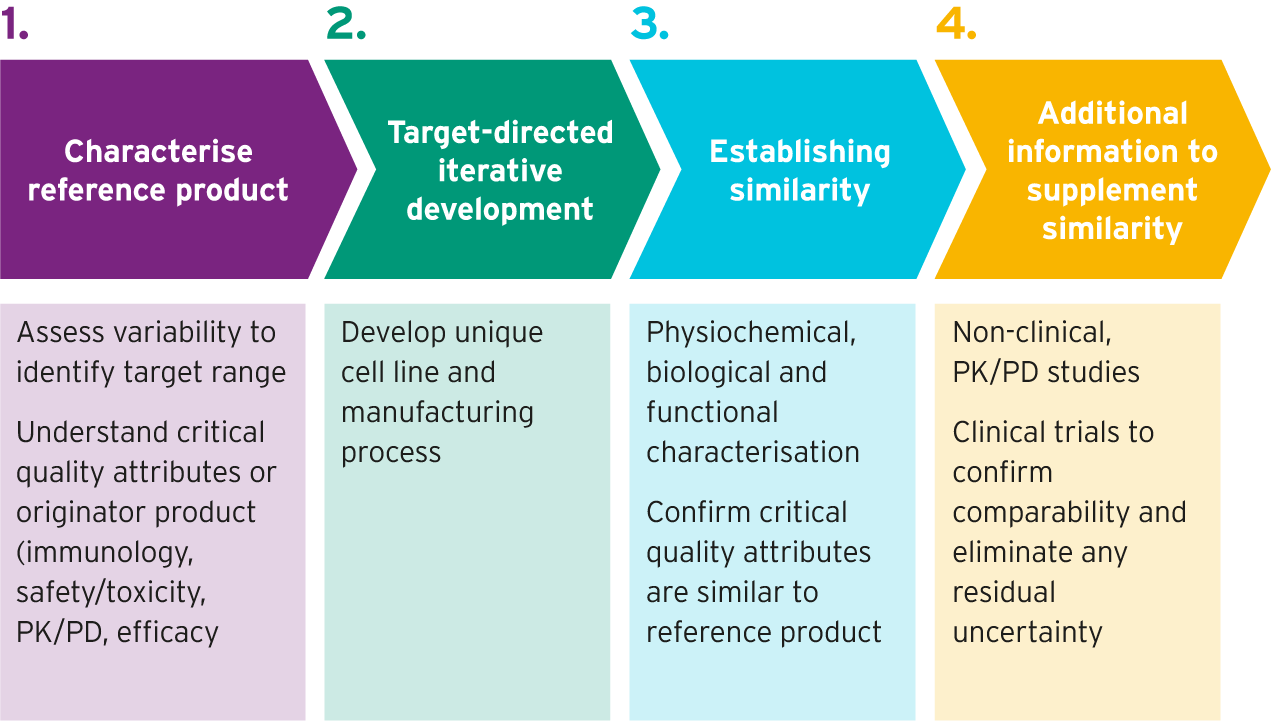
Once the reference product has been characterized, live cells are used to develop biosimilar candidates. Highly trained scientists carry out the following steps to do this:
- They insert genetic instructions for a clone (cell) to produce a specific candidate biologic5
- After different versions of the biologic are made, each one by a different cell, screening is conducted to identify the cell line that produces the protein that is most similar to the reference biologic5
- A process to manufacture the biosimilar is developed, in a way that maximizes the similarity between the biosimilar and the reference product5,6
Biosimilar development process7,8
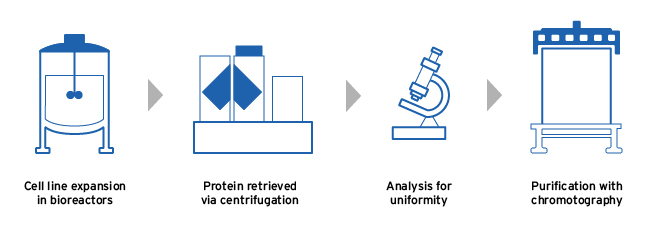
Fresenius Kabi manufactures its biosimilars using the following steps in order to produce a high-quality final product:
- The chosen cell line is expanded in large bioreactors, in conditions optimized for protein production5,6
- The harvested protein is analyzed for uniformity in its 3D structure and potency before being purified5
- The synthesised biologic is isolated and purified, removing any unwanted impurities5,6
Biosimilar manufacturing process5,6
After the manufacturing system has been developed, clinical and non-clinical studies that compare the biosimilar with the reference product can take place.
How are biosimilars approved? Learn here
References and footnote
GMP, good manufacturing practice
1. European Medicines Agency. Biosimilars in the EU: Information guide for healthcare professionals, 2017. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Leaflet/2017/05/WC500226648.pdf (accessed in December 2020). 2. US Food and Drug Administration (FDA) – Quality considerations in demonstrating biosimilarity of a therapeutic protein product to a reference product. 2015. Available at: https://www.fda.gov/downloads/drugs/guidances/ucm291134.pdf (accessed in December 2020). 3. Blauvelt A et al. Brit J Dermatol 2016; 174:282–286. 4. US. Food and Drug Administration (FDA). Guidance for industry Q6B specifications: Test procedures and acceptance criteria for biotechnological/biological products. 1999. Available at: http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm073488.pdf (accessed in December 2020) 5. Gupta SK et al. Opportunities and challenges in biosimilar development. 2017. Available at: http://www.bioprocessintl.com/manufacturing/biosimilars/opportunities-challenges-biosimilar-development/ (accessed in December 2020). 6. Al-Sabbagh A et al. Sem Arth Rheum 2016; 45:S11–S18. 7. McCamish M, Woollett G. Clin Pharmacol Ther 2013; 93:315–317. 8. de Mora F. Br J Clin Pharmacol 2015; 80:949–956.
